Clinical Trials
HLB PHARMA
CTT-004
CTT-004
Taltirelin hydrate is an analog of TRH (Thyrotropin Releasing Hormone).TRH stimulates secretion of Thyroid Stimulating Hormone (TSH), which strengthens the effect of catecholamine, a sympathetic stimulator, leading to an increase in the secretion of neurotransmitters acetylcholine and dopamine, thereby improving ataxia. In addition, it increases serotonin secretion which helps improve judgment, memory, and depression disorders concurrent with ataxia.
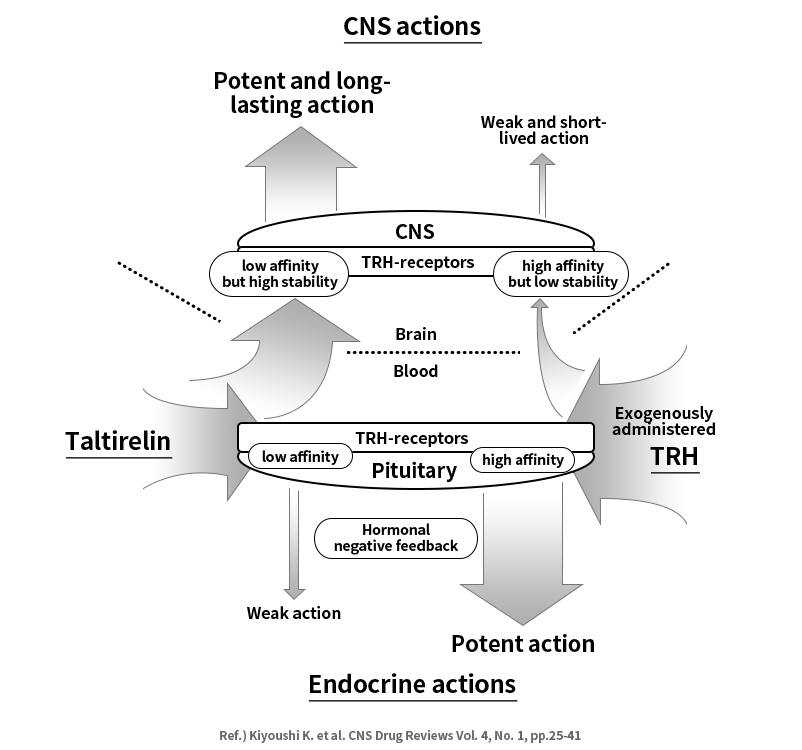


What is spinocerebellar degeneration?
Spinocerebellar degeneration is a rare disease in which the cells making up the cerebellum die due to unknown mechanisms. Ataxia is the main symptom caused by spinocerebellar degeneration. As there has been no therapeutic agent with efficacy and safety proven through large-scale clinical trials, MEDIFORUM is currently carrying out clinical trial that aims to prove efficacy and safety at major domestic university hospitals, using the taltirelin hydrate.
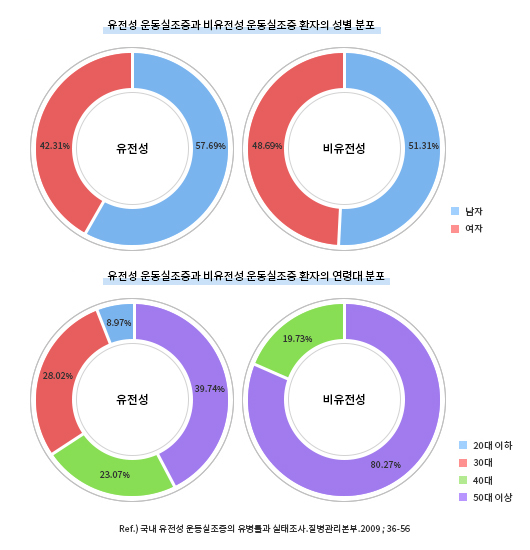
Prevalence
Spinocerebellar degeneration is a rare disease. The prevalence rate of hereditary and non-hereditary ataxia nationwide stands at 8.29 out of 100,000 persons, out of whom the prevalence rate of hereditary ataxia is 4.99 out of 100,000 persons.
In both hereditary and non-hereditary groups, the prevalence rate is higher in men than in women. Hereditary ataxia is distributed widely throughout all age groups while non-hereditary ataxia occurs more often in people over age 50.
CTT-004 clinical overview
| Study Title | A multicenter, randomized, double-blind, placebo-controlled clinical trial to Evaluate and Compare the Safety and Efficacy of C-Trelin OD Tablet 5mg(Taltirelin Hydrate) in Patients with Ataxia Induced by Spinocerebellar Degeneration |
|---|---|
| Study Indication | Ataxia Induced by spinocerebellar degeneration |
| Investigational Product | C-Trelin OD Tablet 5mg(Taltirelin Hydrate) |
| Targeted subject No. | 166participants(Clinical trials currently underway in 8 university hospitals) |
| Study Period | 24 months after the date of IRB approval |
| Study Purpose | Evaluation and establishment of the efficacy in patients with ataxia Induced by spinocerebellar degeneration |


